about the studies
Precision-HD is the first study that uses drugs called ASOs, which are now in multiple clinical trials in Huntington’s disease, aiming to lower production of the harmful mutant huntingtin protein in the brain through a "allele-selective" approach targeting genetic mutation that causes Huntington’s disease. It is a global clinical program consisting of the PRECISION-HD1 trial evaluating investigational WVE-120101 targeting SNP1 and the PRECISION-HD2 trial evaluating investigational WVE-120102 targeting SNP2.
Two parallel, multicenter, double-blind, randomized, placebo-controlled Phase 1b/2a clinical trials for WVE-120101 and WVE-120102, administered intrathecally, with single-ascending dose and multipleascending dose portions. The Primary objective of those clinical trial is to assess safety and tolerability of intrathecal doses in early manifest HD patients
Sponsor: Wave Life Sciences
about the experimental drugs
Wave’s ASO drugs aim to target just the mutant version of the huntingtin protein, leaving wild-type production relatively unaltered.
SNPs are a common type of genetic variation that normally occur in all humans, but may also act as biological markers to aid in locating genes associated with a particular disease. Previous HD research has identified multiple SNPs that are associated with the disease-causing expanded cytosine-adenine-guanine (CAG) repeat, which is an abnormality present in all HD patients that results in the production of mutant huntingtin protein, and causes HD. Therefore, Wave is utilizing common SNPs to precisely target the underlying cause of the disease.
Information about the functioning of our cells is contained in the DNA. DNA must be transcribed into messenger RNA so that this information can then be translated by the ribosomes used to "make" proteins. The antisense drug is a small piece of DNA that recognizes and binds to messenger RNA, blocking its translation into protein. In people with the mutated huntingtin gene (mHTT), ASOs are designed to reduce the production of the messenger RNA that tells cells to produce mHTT.
about asos
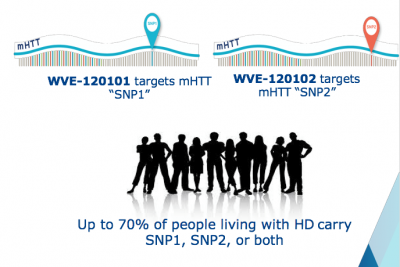
WVE-120101 and WVE-120102 (ASO) are designed to selectively target mutant huntingtin (mHTT) mRNA transcription early manifest HD who carry a SNP at the rs362307 (“SNP1”) or the rs362331 (“SNP2”) location, respectively.
SNPs, or single nucleotide polymorphisms, are a kind of 'genetic fingerprint' present in DNA and they help to recognize specific points of a gene and its mRNA. It is as if the SNPs represent a pin on a map (in this case the mHTT) that allows the drug to identify and target, reducing it, the defective protein instead of the healthy one.
SNP1 and SNP2 are possessed by 70% of the patients.
study sites
Precision - HD 1
It involves 20 sites in the following countries: Australia, Canada, Denmark, Poland, United Kingdom
Number of participants: 60
Started in July 2017 - estimated end date December 2020
Precision - HD 2
It involves 20 sites in the following countries: Australia, Canada, Denmark, France, Germany, Poland, United Kingdom
Number of participants: 60
Started in July 2017 - estimated completion: December 2020
drug administration
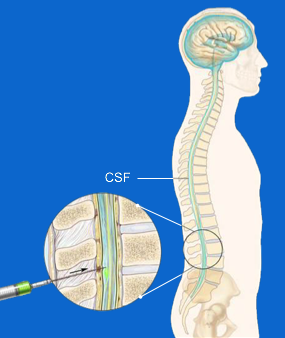
The ASOs are not able to reach the brain directly due to the existence of a sort of 'defense wall' that our body produces around the brain itself, which is called the 'blood brain barrier'. This means that in order to reach the brain they have to be infiltrated in some other way. Both WVE-120101 and WVE-120102 are administered Intrathecally in patients with Huntington's Disease.
inclusion criteria
Symptomatic adult patients with early manifest Huntington's disease (HD) can participate.
Specific inclusion criteria are:
- Prescreened with targeted SNP on the same allele as the pathogenic CAG expansion
- Male or female patients aged ≥25 - ≤65 years
- Clinical diagnostic motor features of HD, defined as Unified Huntington's Disease Rating Scale (UHDRS) Diagnostic Confidence Score = 4
- Early manifest HD, Stage I or Stage II based on UHDRS Total Functional Capacity Scores ≥7 and ≤13
Phase 1b / 2a will be completed successfully if ...
Whether the drugs are safe and well tolerated throughout the study and whether the levels of mHTT dosed in the CSF are effectively reduced. In this case, a subsequent phase III will be launched on a larger number of patients to assess the efficacy, or the clinical benefit, of the two molecules on patients.
To know the details of the two studies consult:

